Paxlovid
Researchers at Mass General Brigham found Pfizers Paxlovid can cut the risk of hospitalization and death by nearly half among those who are vaccinated. Paxlovid nirmatrelvir tablets co-packaged with ritonavir tablets has a Emergency Use Authorisation EUA from the FDA to treat mild-to-moderate COVID-19 in adults and children.

Paxlovid In Eu Zugelassen Was Bringt Das Corona Medikament Nachrichten Wdr
In clinical trials the antiviral was associated with an.

. Pfizer Paxlovids maker has declined to share its pricing plan but the company has announced that its COVID vaccine will cost 120 per dose when it is no longer provided by the. It is important you start treatment as soon as. 1 It has demonstrated.
Its used to treat early COVID-19 infection. Scott Dryden-Peterson one of. Paxlovid is an oral antiviral treatment that can be taken at home to prevent high-risk COVID-19 patients from becoming sick enough to be hospitalized.
Paxlovid is given to people who are at most risk of becoming very unwell from a COVID-19 virus infection. Paxlovid the antiviral pill produced by Pfizer has helped prevent many people infected with COVID-19 from being hospitalized or dying. The antiviral drug Paxlovid has the potential to significantly reduce the mortality rate associated with Covid-19 but is underused by those who would benefit most.
Paxlovid is still one of only a few COVID-19 antiviral treatments authorized by the FDA others include a pill called molnupiravir and remdesivir an IV therapy so people are still asking for it. Ritonavir may also increase blood concentrations of certain concomitant medications. A recent CDC report notes.
Overall the drug works as promised doctors say by dramatically reducing the chances that an. 13 2022 1257 pm. It may help you become less sick and stay out of hospital.
Nirmatrelvir is an oral protease inhibitor that is active against M PRO a viral protease that plays an essential role in viral replication by cleaving the 2 viral polyproteins. Paxlovid is an antiviral medicine that works by stopping the virus that causes coronavirus COVID-19 from growing and spreading in the body. Developed by Pfizer a clinical trial.
People can purchase the medication on the app if. Government plans to stop footing the. Paxlovid an oral antiviral medication will start to work against COVID as soon as you take it but you may not start to feel better right away.
Paxlovid is a prescription antiviral medicine used to treat the symptoms of COVID-19 with emergency use authorization EUA. Because ritonavir-boosted nirmatrelvir Paxlovid is the only highly effective oral antiviral for the. Paxlovid nirmatrelvirPF-07321332 and ritonavir is an oral antiviral drug that should be initiated as soon as possible after diagnosis of COVID-19 and within 5 days of symptom onset.
Paxlovid helps older people survive COVID but younger healthy people see little benefit. Paxlovid nirmatrelvirritonavir is an oral antiviral medication used to treat COVID-19 in people at high risk for severe illness. Paxlovid is an oral antiviral pill that can be taken at home to help keep high-risk patients from getting so sick that they need to be hospitalized.
Paxlovid the drug used to treat COVID-19 infections reduced hospitalizations and death in a highly vaccinated population of adults over the age of 50 by 44 percent according to a new. WASHINGTON - The US government agreed to pay Pfizer nearly US2 billion S27 billion for an additional 37 million courses of its Covid-19 antiviral treatment Paxlovid the company said. So if you test positive for the coronavirus and.
It consists of a 5-day course of. Healthcare company 111inc has started selling Paxlovid for 2980 yuan 42680 per box the sales page on the app showed on Tuesday. Government has so far purchased 20 million courses of Paxlovid priced at about 530 each a discount for buying in bulk that Pfizer CEO Albert Bourla called really very attractive.
The most common side effects of Paxlovid include. Paxlovid nirmatrelvirritonavir is an oral COVID-19 treatment that certain people can take if they test positive for the virus and have mild to moderate symptoms. Reports of Paxlovid rebound popped up in the spring of 2022 as some people who had taken the antiviral medication experienced a recurrence of Covid.
It has a few known side effects but most are mild in nature. The antiviral drug nirmatrelvir plus ritonavir Paxlovid was granted Emergency Use Authorization for treating COVID-19 in December 2021.
Pfizer To Deliver Up To Six Million Courses Of Paxlovid To Global Fund

Corona Studie Aus Israel Paxlovid Zeigt Offenbar Hohe Wirksamkeit Bei Uber 65 Jahrigen

Paxlovid Informationen Zur Direktabgabe
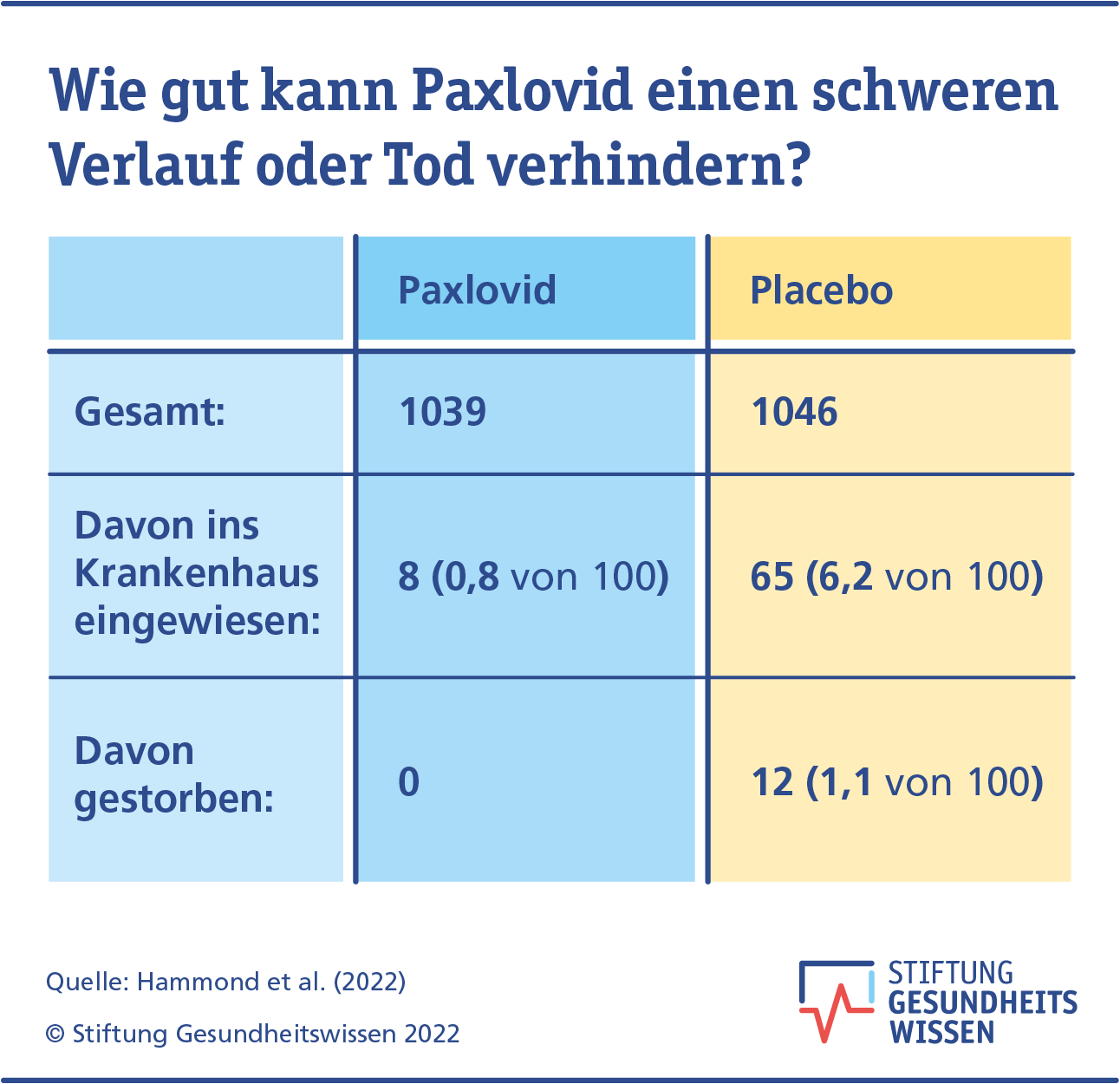
Paxlovid Wie Wirksam Und Sicher Ist Das Covid Medikament Stiftung Gesundheitswissen
Paxlovid As A Covid 19 Treatment What You Should Know Time

Paxlovid Hunderttausenden Packungen Droht Vernichtung

Paxlovid Startet Auf Dem Deutschen Markt Pta In Love

Hausarzte Konnen Paxlovid Direkt Abgeben Regelung Gilt Vorerst Bis Ende September

13 Things To Know About Paxlovid The Latest Covid 19 Pill News Yale Medicine

Patienten Uber Paxlovid Aufklaren
Paxlovid Bei Covid 19 Cochrane Deutschland

Arzte Verschreiben Corona Medikament Paxlovid Nur Selten

Paxlovid Fur Wen Das Corona Medikament Infrage Kommt Apotheken Umschau

Hoffnungstrager In Der Corona Therapie Paxlovid Neues Covid Medikament Rbb
/cloudfront-us-east-2.images.arcpublishing.com/reuters/TW2OI4E77NNATO5MNXA7ITAOAA.jpg)
China Approves Use Of Pfizer S Covid Drug Paxlovid Reuters

Paxlovid Hhs Aspr

Paxlovid Wie Wirksam Und Sicher Ist Das Covid Medikament Stiftung Gesundheitswissen